COVID-19 Rapid Antigen Test (3 Pack)
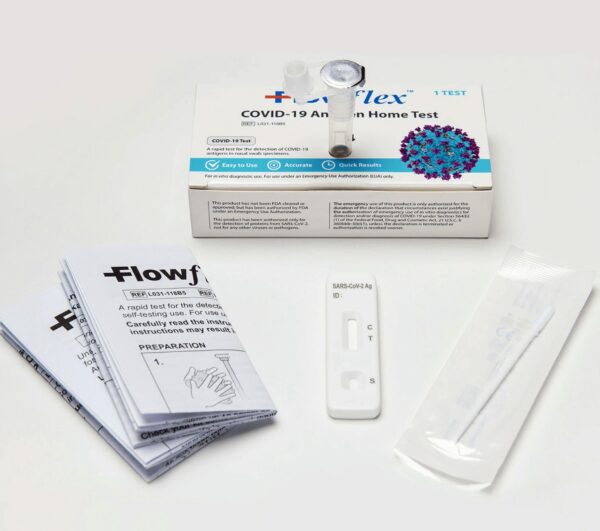
Pack of 3 Flowflex COVID-19 Official Emergency Use Authorized 15 minute rapid antigen test kits. Each order placed includes 3 individually boxed genuine COVID-19 antigen tests. Ships within 24 hours of order placement. Guaranteed Genuine
Original price was: $39.99.$9.99Current price is: $9.99. — or subscribe and save up to 15%
Out of stock

Orders over $75 ship fast and free!
Shipped Directly from our Warehouse!

Satisfaction 100% Guaranteed!
COVID-19 Rapid Antigen Test Kit – FlowFlex – 3 Individually Boxed Tests
Each test is individually packaged in its own box
Find peace of mind with Flowflex!
- Easy-to-use nasal swab test
- Requires just 1 test*
- Can be used to test children as young as 2 years old
- For use with and without COVID-19 symptoms
- Accurate results in 15 minutes
- No need to send off to a lab to obtain results
- Compact packaging for “On-The-Go” testing
*Other COVID-19 antigen home tests may require a 2nd test 2-3 days after the first.
Get Back to Sharing Special Moments
with Family and Friends
The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s COVID-19 status.
Critical Information When & Where You Need It
The Flowflex™ COVID-19 Antigen Home Test is all you need to determine your family’s Covid-19 status, whether symptoms are present or not. Can be used on children as young as 2 years old. Get the convenience of Flowflex!
- Easy and Affordable
- Highly Accurate Nasal Swab Test
- Quick Results in 15 minutes
- Safe for children as young as 2 years old
- For use with and without symptoms
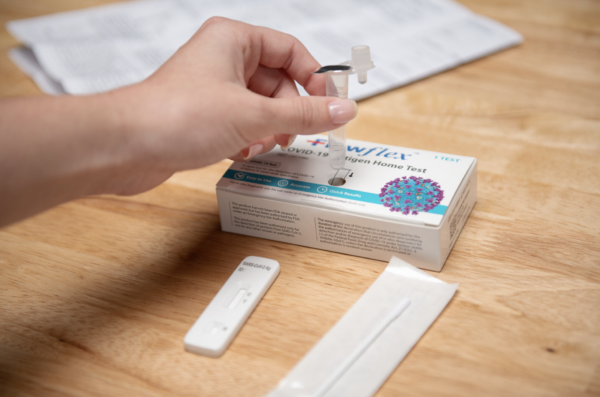
Test Procedure Overview
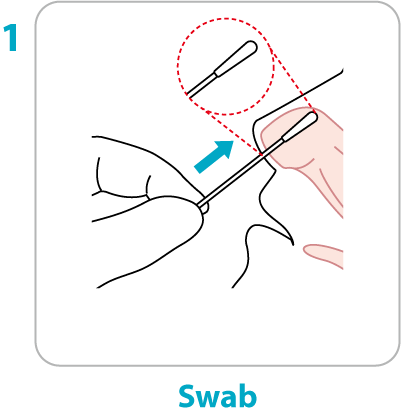
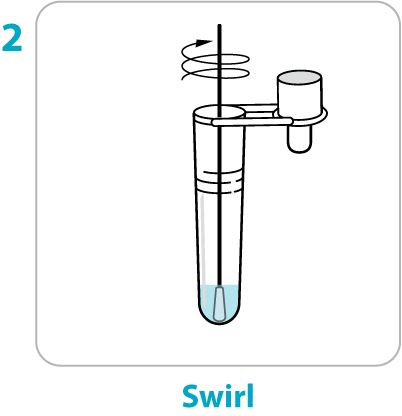
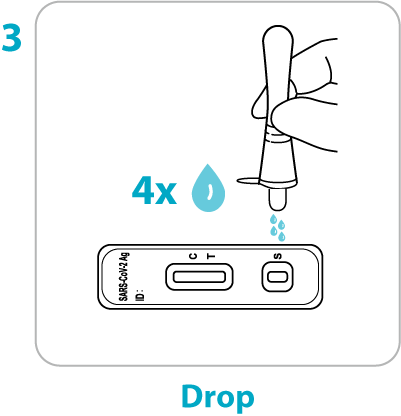

This test procedure overview does not replace the package insert. Before you begin the test, it is important to read and follow the detailed instructions in the package insert.
This product has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVDs for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner.

US Physician Owned Small Business.